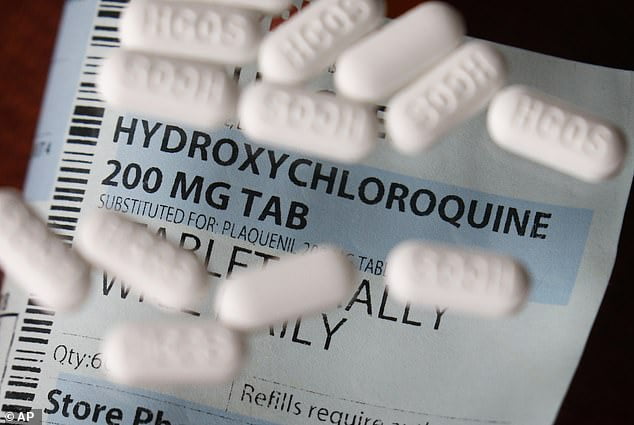
The anti-malarial drug hydroxychloroquine, which President Donald Trump has touted as a treatment for COVID-19, was found to have no benefits and linked to higher death rates in coronavirus patients, according to a new study.
The study, which was posted Tuesday on medrxiv.org, analyzed the outcomes of 368 male Veterans Affairs patients hospitalized with COVID-19 nationwide until April 11. The study hasn’t been peer-reviewed and was funded by the National Institutes of Health and the University of Virginia.
Of the patients studied, 97 received hydroxychloroquine, 113 received hydroxychloroquine with the antibiotic drug azithromycin, and 158 didn’t receive any hydroxychloroquine, according to the study.
More than 27% of patients treated with hydroxychloroquine died and 22% treated with both hydroxychloroquine and azithromycin died, compared to the 11.4% who died and weren’t treated with those drugs, according to the study.
“An association of increased overall mortality was identified in patients treated with hydroxychloroquine alone. These findings highlight the importance of awaiting the results of ongoing prospective, randomized, controlled studies before widespread adoption of these drugs,” the authors of the study wrote.
The study also found that hydroxychloroquine didn’t reduce the risk of a COVID-19 patient having to go on a ventilator.
Other drugs and treatments are still going through clinical trials, media outlets reported.
Trump has said hydroxychloroquine has “tremendous promise” as a treatment for COVID-19, CNN reported.
“I think it’s going to be great,” he said, according to CNN. “We’re quickly studying this drug.”
But what about other treatments for the coronavirus?
The preliminary information from two clinical trials for antiviral drug remdesivir has been promising for COVID-19 treatment, according to U.S. News & World Report.
“Early results are promising, and that is important right now. Much of what we are learning about COVID-19 management is centered around preventing quick deterioration. Timing is everything. I can’t say for certain they [patients] would have been intubated otherwise, but it’s encouraging,” Katherine Perez, an infectious diseases pharmacist who is co-leading the trials, told U.S. News and & World Report.
Some of the patients who took remdesivir have recovered and others have been released from the hospital, according to U.S. News and & World Report. Researchers also say the drug could reduce the rate at which patients have to be put on ventilators, according to the outlet.
Convalescent plasma — which is a treatment that works by taking the donated blood from recovered coronavirus patients and seeing if the antibodies can fight the virus in another person — is still being implemented across the country, ABC News reported.
The Food and Drug Administration’s Emergency Investigational New Drug process led to the first convalescent plasma treatments to be performed in New York and Texas in late March, according to ABC. Doctors say they are monitoring the treatment and the effectiveness will be determined from clinical trials.






2 Comments