The US is buying nearly all the next three months’ projected production of Covid-19 treatment remdesivir from US manufacturer Gilead.
The US health department announced on Tuesday it had agreed to buy 500,000 doses for use in American hospitals.
Tests suggest remdesivir cuts recovery times, though it is not yet clear if it improves survival rates.
Gilead did sign a licensing deal in May for production outside the US but it is still in its early stages.
“President Trump has struck an amazing deal to ensure Americans have access to the first authorised therapeutic for Covid-19,” Department of Health and Human Services Secretary Alex Azar said in a statement.
A course of treatment in the US will cost $2,340 (£1,900).
Nine companies can make the drug under licence outside the US for distribution in 127 mostly poorer countries, and the cost is lower. But the project is still in its early stages.
Additional quantities are being manufactured for use in clinical trials.
But critics say the US move to buy up so much stock from Gilead itself undermines international co-operation on Covid, given that other countries have taken part in trials of remdesivir, originally an anti-viral against Ebola.
“The trial that gave the result that allowed remdesivir to sell their drug wasn’t just done in the US. There were patients participating through other European countries, in the UK as well, and internationally, Mexico and other places,” Oxford University’s Prof Peter Horby told BBC Radio 4.
He said the move also had implications for any possible future vaccine, with the need for “a much stronger framework if we are going to develop these things and they’re going to be used for national emergencies”.
Senior Sussex University lecturer, Ohid Yaqub, said: “It so clearly signals an unwillingness to co-operate with other countries and the chilling effect this has on international agreements about intellectual property rights.”
Some in the US have criticised the purchase price, as taxpayer money had helped fund remdesivir’s development.
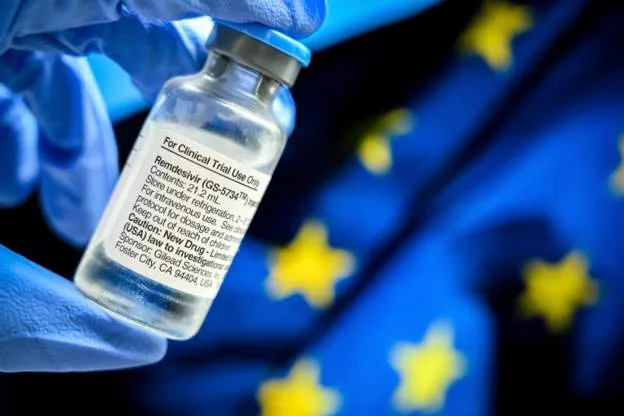
Short supply
While there is international concern about the US buying up almost all supplies of remdesivir, we must remember that Gilead is a US company, and that the country has recorded the highest number of cases and deaths from Covid-19.
Under US law, export of drugs considered essential to treatment of patients in a public health emergency can be banned – and many other countries, including the UK, have similar legal provisions.
Gilead has put in place voluntary licensing agreements with a number of manufacturers around the world with the aim of producing the drug for use in developing nations.
In extremis, there is also a compulsory licensing mechanism which could allow countries to ignore Gilead’s intellectual property rights, and manufacture their own generic versions of the drug.
However, the US deal will inevitably mean that for the next three months at least the drug – one of only two that have so far been of proven benefit for patients who are seriously ill with Covid-19 – will be in short supply in many other countries. The UK, however, says it currently has sufficient stocks for patients who need it.
The US is among several countries to approve remdesivir for use in combating Covid-19.
The European Union is expected to give approval this week, according to Germany’s health ministry.
Germany says it has sufficient supplies of the drug and expects to be able to acquire more from Gilead in the future.
In the UK, the Department of Health said it had enough to treat every National Health Service patient who needed it.
BBC





2 Comments